Steps to CE Marking
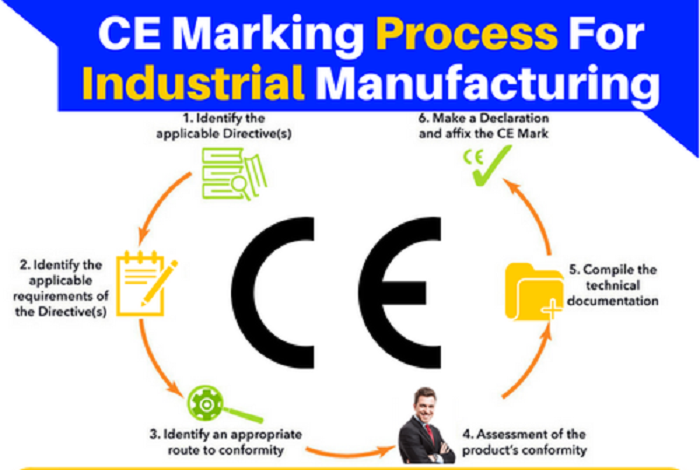
STEP 1: IDENTIFY THE APPLICABLE DIRECTIVE(S)
The first step is to identify whether your product can be CE marked or not. Not all products are required to be CE Marked, only the products that fall within the scope of at least one of the CE Marking Directives. There are more than 20 product Directives & Regulations covering a range of products. Such products includes (but not limited to) electrical equipment, machines, medical devices, toys, pressure equipment, PPE, wireless devices and construction products.
STEP 2: IDENTIFY THE APPLICABLE REQUIREMENTS OF THE DIRECTIVE(S)
Photo of a standard
Each Directive has slightly different methods of demonstrating conformity. This usually depends on the classification of the product and its intended use. Every Directive has a number of ‘essential requirements’ which the product has to meet.
The best way to demonstrate that these essential requirements have been met is by meeting the requirements of applicable ‘harmonised European Norms’ (Standard) known as hENs. Standards may offer a presumption of conformity to the essential requirements of the applicable Legislation. However do not forget that the use of standards usually remains voluntary. hENs can be identified by searching the ‘official journal’ on the European Commission’s website.
STEP 3: IDENTIFY AN APPROPRIATE ROUTE TO CONFORMITY
The CE Marking process is always a self-declaration process however you may need to involve a third party. This is set out in the ‘system of attestation’ and will vary between Directive. Some products (such as invasive medical devices or fire alarm systems) may, to some extent, have a mandatory requirement for some involvement of an authorised third party.
STEP 4: ASSESSMENT OF THE PRODUCT’S CONFORMITY
When all of the requirements have been established, you need evidence that the product meets the essential requirements of the Directive(s).
This usually involves some assessment and/or testing. It will often involve ensuring that the requirements of the applicable harmonised standard(s), which were identified in step 2, have been met.
STEP 5: COMPILE THE TECHNICAL DOCUMENTATION
Technical documentation relating to the product or range of products needs to be compiled. This information should cover every aspect relating to conformity and is likely to include details of the design, development and manufacture of the product. Technical documentation may also be known as the Technical File or Technical Construction File.
- Technical description.
- Drawings, circuit diagrams and photos.
- Bill of materials.
- Specification and, where applicable, Declarations of Conformity for the critical components and materials used.
- Details of any design calculations.
- Test reports and/or assessments.
- Instructions.
- Copy of the Declaration.
Technical documentation can be made available in any format (i.e. paper or electronic) and must be held for a period of up to 10 years after the manufacture of the last unit, and in most cases reside in the European Economic Area (EEA).
STEP 6: MAKE A DECLARATION AND AFFIX THE CE MARK
When the manufacturer, importer or authorised representative is satisfied that their product conforms to the applicable CE Marking Directives, they must complete a Declaration. Under most Directives it is known as the EU Declaration of Conformity but other terms exist. Such as Declaration of Incorporation for partly completed machinery and Declaration of Performance for construction products.
>>> Watch now: European standard